Product compliance management is first and foremost a question of efficient, up-to-date and traceable document management. This becomes particularly clear with series products that are sold over a longer period of time.
We want to describe this problem and its requirements using a very specific example. This will make some of the relationships clearer and illustrate the importance of good document management. We will also deliberately take different perspectives to illustrate what requirements are placed on products by consumers and authorities and who has to fulfill them and how.
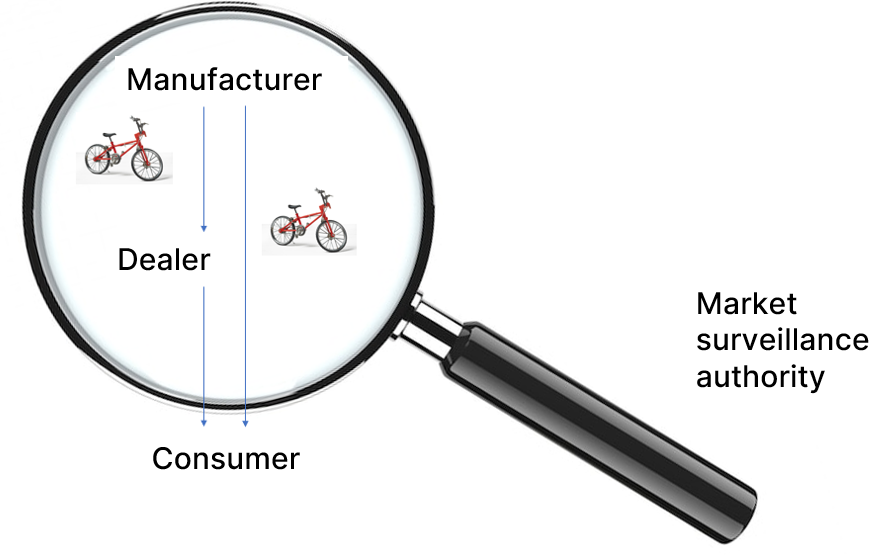
In business administration, a product is a tangible good (or an intangible service) that is the result of a production process. In other words, a product often consists of several parts. These different parts can each be a product in their own right.
I buy a bicycle from my trusted dealer or from the manufacturer’s online store. I have purchased the product “bicycle”. But if I take a closer look at it, I will notice that the bike has a saddle, rims, tires, a bell, brakes, perhaps an electric motor and much more.
Many of these parts could also be bought separately, making them stand-alone products, and sometimes you see these identical parts on bicycles from other manufacturers (e.g. the tires or the electric motor).
The bike I want to buy should appeal to me, meet my individual needs, be safe to operate and be equipped in accordance with the applicable regulations. That’s it! Oh yes; the price should also be right.
Market surveillance authorities are primarily concerned with the safety of a product. This primarily includes mechanical, electrical and chemical risks, but increasingly also requirements that products must fulfill for ecological reasons and objectives (e.g. RoHS).
An official EU website states: “In the technical documentation, you provide information on the design, manufacture and operation of your product. It must contain all the information necessary to demonstrate the conformity of the product with the applicable regulations”
As a manufacturer, you must comply with various regulations – depending on the product in question – if you wish to sell a product. You must draw up technical documentation before placing the product on the market and ensure that the technical documentation can be made available to the market surveillance authorities.
You must keep this technical documentation for ten years from the date you place your product on the market (unless explicitly stated otherwise).
The technical documentation is required to prove that the product meets the essential requirements. It therefore serves to justify and substantiate the EU Declaration of Conformity, which is mandatory for products subject to CE marking.
You also need this documentation in order to be able to affix the CE marking to your product. By affixing the CE marking, the manufacturer promises to have complied with all relevant applicable harmonized Union regulations.
The above information applies to products manufactured in the EU. If you import products from non-EU countries, you are to be regarded as the distributor in the eyes of the authorities in the same way as the manufacturer. You therefore assume full responsibility for the products as well as the obligation to prepare or submit the complete technical documentation.
Let’s stay with the bicycle. Before I place my product on the market or make it available on the market, I have the task of informing myself about the applicable requirements and providing evidence that these requirements have been met.
This evidence should be provided in the form of documents (electronically or on paper) so that authorities can view and check them if necessary. These documents should be based on test reports from independent test institutes and should not be too old – but more on the age of the documents later.
A large number of documents are usually already available during the development process, such as test reports for the materials used, data sheets for electronic components or even the BOM (Bill of Material). It is therefore advisable and efficient to integrate the proof of conformity into your product development right from the start.
The production process should also be included, and I will come back to this later – keyword: component modification during production.
It should not be forgotten at this point: the packaging is also part of the product and this must also meet the legal requirements and be compliant (e.g. with regard to compliance with certain pollutant limits).
So far so good: it may be time-consuming to create this documentation, but it is anything but rocket science. And it is essential if you want to sell your products in compliance with the law.
Here is a brief (non-exhaustive) list of the requirements for a product. What you need:
So, it’s done!
As a manufacturer, I now have my complete set of documents and my product is produced exactly as described. There have also been no component changes during production. My product is ready to be made available on the market.
It can be sold!
Another small note: even if my product is not sold because it is, for example, a promotional gift, it is considered to be made available on the market and must therefore also be compliant!
Unfortunately, the responsibility of the manufacturer or distributor is not yet fulfilled. The time of first placing on the market is merely a snapshot. The manufacturer is responsible for the entire life cycle of the product.
With products that are sold on the market for a long time (series production), sooner or later situations arise that make it necessary to update the documentation. We take our bicycle again.
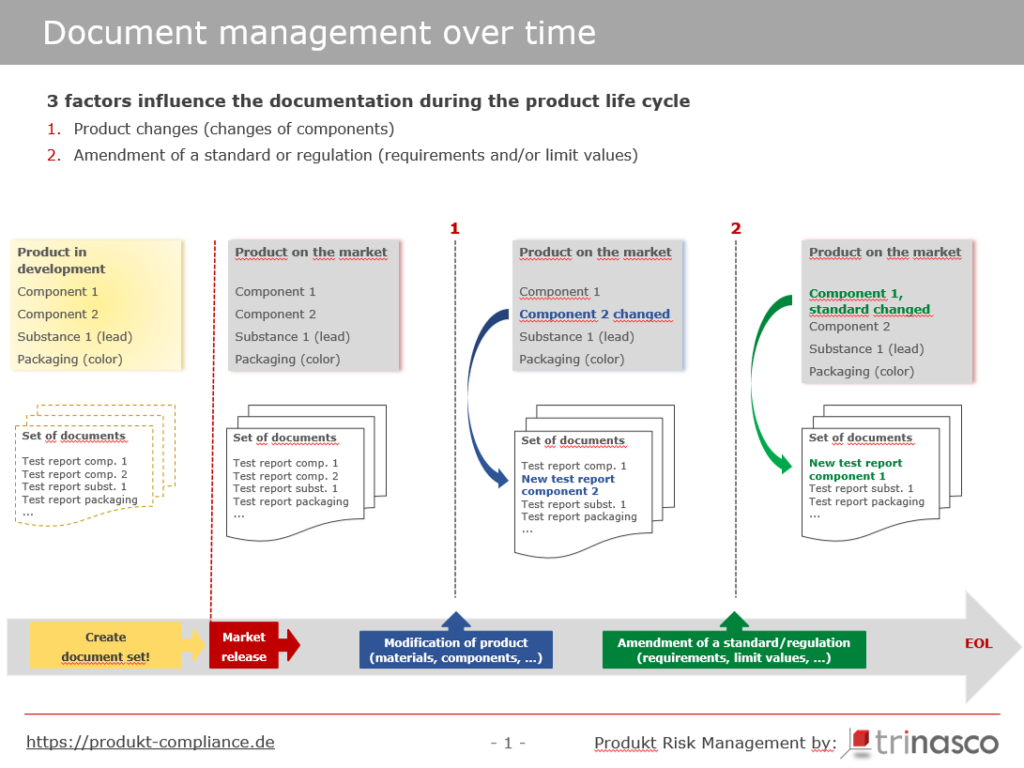
Let’s take a closer look at Figure 3. At the time of first placing on the market (market release), the manufacturer has prepared complete documentation, carried out risk analyses, commissioned mechanical and chemical tests and affixed the correct markings to the product and packaging.
The documentation consists of analyses and tests from independent testing institutes that the manufacturer has commissioned himself and/or documents that his suppliers have made available to him.
After one year, however, the supplier of the tires has changed its rubber compound (technical progress, cost savings) and will no longer supply the “old” tires (Figure 3: blue dashed line No.1: change to the product). The supplier had provided the manufacturer with a document confirming compliance with the legal requirements for the old tires.
For example, its old tires did not contain critical plasticizers exceeding 0.1% by weight, which would have required the manufacturer to notify its distributors and add the entire bicycle to the SCIP database.
As a result of the change to the component, the manufacturer requires a new document from its supplier confirming that the new tires also comply with the legal requirements. The manufacturer may also have the tires tested by an independent laboratory to ensure compliance with legal regulations.
Depending on the component and its modification, the entire product may have to be retested. For example, when replacing a motor in an electrical product, certain regulations (e.g. the Low Voltage Directive, EMC) may have to be checked not only for this component, but for the entire product.
For example, if the new motor of a washing machine has completely new performance parameters, the electromagnetic phenomena are also likely to change, making it necessary to test the entire machine. If the seal is changed or smaller components are modified, a new complete test does not usually have to be carried out.
In a similar way, the replacement of a component can also lead to a new risk analysis having to be carried out, as the new component leads to new risks that were not considered in the original risk analysis.
For example, replacing the halogen lamp previously installed in a bicycle with an LED lamp leads to a new risk assessment. The blue light risk must now be taken into account in the risk analysis and tested in accordance with EN 62471 – a risk that does not exist in this form with halogen lamps.
Another practical example: during the coronavirus pandemic, capacitors used in computer power supplies became scarce and were often replaced by cheaper versions. In this case, the risk analysis would have had to be carried out again and the entire product would have had to be retested in each case to ensure the suitability of the component before installation.
In any case, the manufacturer should adapt the document set to the new situation and create a new document set. Ideally, he should also record the exact period of time affected by the product change and, if necessary, even change the article number of the product or add a suffix. A preferred means of traceability is the use of serial or batch numbers.
In the event of an inspection by the authorities or a serious defect in the product (keyword: product recall), the supplier can narrow down the affected products more quickly and avoid unnecessary costs.
The need to carry out new tests and update the document set can also arise because a legal regulation or a standard selected in the testing process has changed (Figure 3: green dashed line No. 2: change to a standard/regulation).
A good example of this is the regular amendment or addition to the so-called candidate list. As a rule, the European Chemicals Agency ECHA expands the candidate list twice a year and supplements the list of substances of concern, about which the recipient must be informed if the limit value of generally 0.1% by weight is exceeded.
If a substance newly added to the list may be contained in the frame of the bicycle, the saddle or the grips, the supplier or the manufacturer should have this checked and prove its safety by means of a test (or other plausible proof) for new deliveries in a new document.
A similar situation can also arise due to the amendment of a CE directive or the expiry of transitional periods. For example, Delegated Directive (EU) 2015/863, often incorrectly referred to as RoHS 3, stipulated in 2015 that a restriction on certain phthalates (DEHP, BBP, DBP and DIBP) will also apply to cables from July 22, 2019.
Since then, manufacturers of electrical products or separate cables must prove that they do not exceed the limits defined under RoHS.
Such changes mean that manufacturers and importers must adapt or update their documentation, even if nothing has changed in their product.
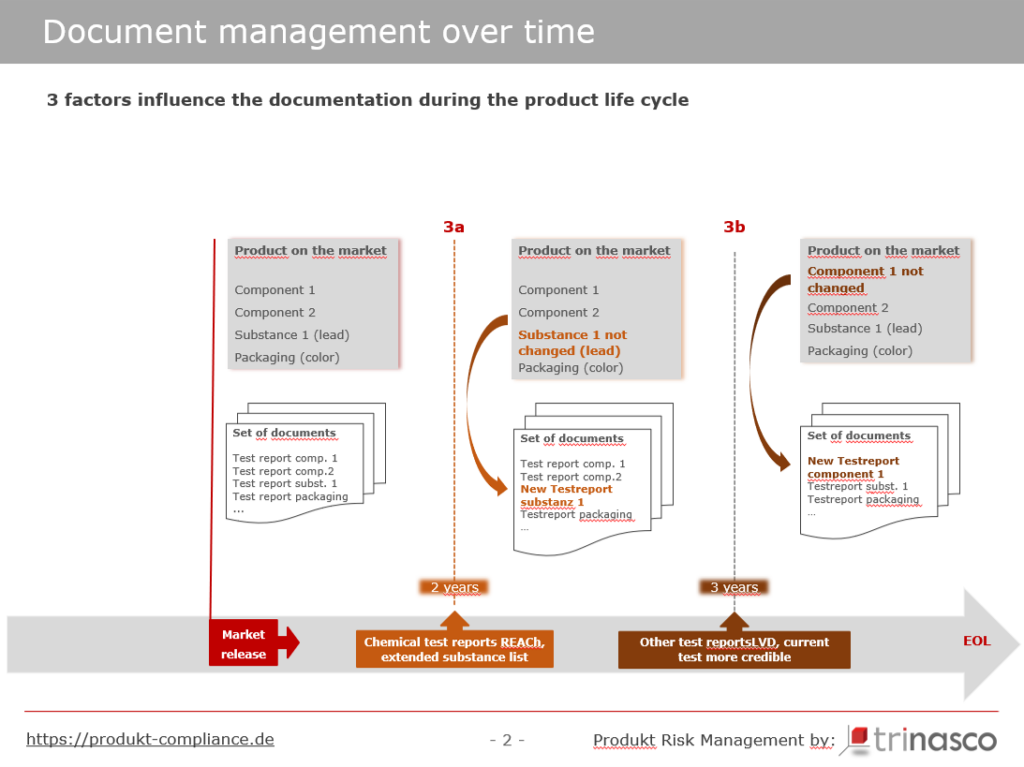
Even if there have been no legal changes for the respective product and the product has remained unchanged in all its components, it makes sense to review and update the documentation after a certain period of time.
Let’s take a look at Figure 4
Many non-food consumer goods have more or less large plastic components, for which documents from chemical tests are usually available. However, as suppliers hardly ever process identical granulates from the same suppliers in exactly the same mixing ratios over a long period of time, chemical test reports are no longer appropriate after approx. 2 years.
To our knowledge, there is no legal requirement for test reports or certificates to be up to date, but we recommend updating such documents after 2 years, even if there have been no changes to the candidate list that could be critical for the product or material in question (Figure 4: orange dashed line 3a – chemical test reports).
For electrical products, we also recommend repeating tests of the products or components after 3 years at the latest and renewing the documents based on them. (Figure 4: brown dashed line 3b – Other test reports)
Even if the components have not changed, which is extremely unusual given the fast-moving nature of electrical components, and no standards and regulations have been adapted, data records that are several years old do not inspire much confidence in customers and authorities.
As can be clearly seen, the initial compilation of a very good (compliant) set of documents is unfortunately only the beginning of comprehensive product compliance management. The technical documentation must be kept up to date throughout the entire product life cycle and ideally be available at the touch of a button.
The larger the product range and the more diverse the products, the more time-consuming this work becomes, especially as companies have to monitor legal and normative changes and changes to the product design for all products.
In our opinion, there are only two basic solutions for this situation:
We would be happy to explain how this could work in your company in a personal meeting.
trinasco GmbH has been advising and supporting manufacturers, importers and trading companies in all areas of product safety and product compliance since 2011. Our activities focus on durable consumer goods and capital goods in all European countries (EU plus EFTA).
In over 100 projects, we have gained extensive experience in various industries, product areas and legal/technical requirements and have a very precise understanding of the content-related, process-related and resource-related challenges in this specialist area.
We have uncovered numerous optimization potentials for our clients in these projects and drastically reduced the financial risks of import or sales bans, product recalls or fines through our work.
As the two founders and managing directors of trinasco themselves have many years of management experience at leading companies in the consumer electronics and food industries (Bose, Nokia, Sony, Pepsi-Cola), we know the internal priorities and possible resistance and can also provide you with optimum support and guidance in the necessary change processes (structures, processes, resources, cooperation with suppliers, etc.).
In order to be able to answer special or detailed questions, we have also been working with experts from related fields for many years and can therefore offer our customers a comprehensive package of solutions.
We help to find and maintain the balance between product compliance and commercial success.